Medical Center stays on top of IV shortage
Hurricane Maria damages Puerto Rico drug manufacturers
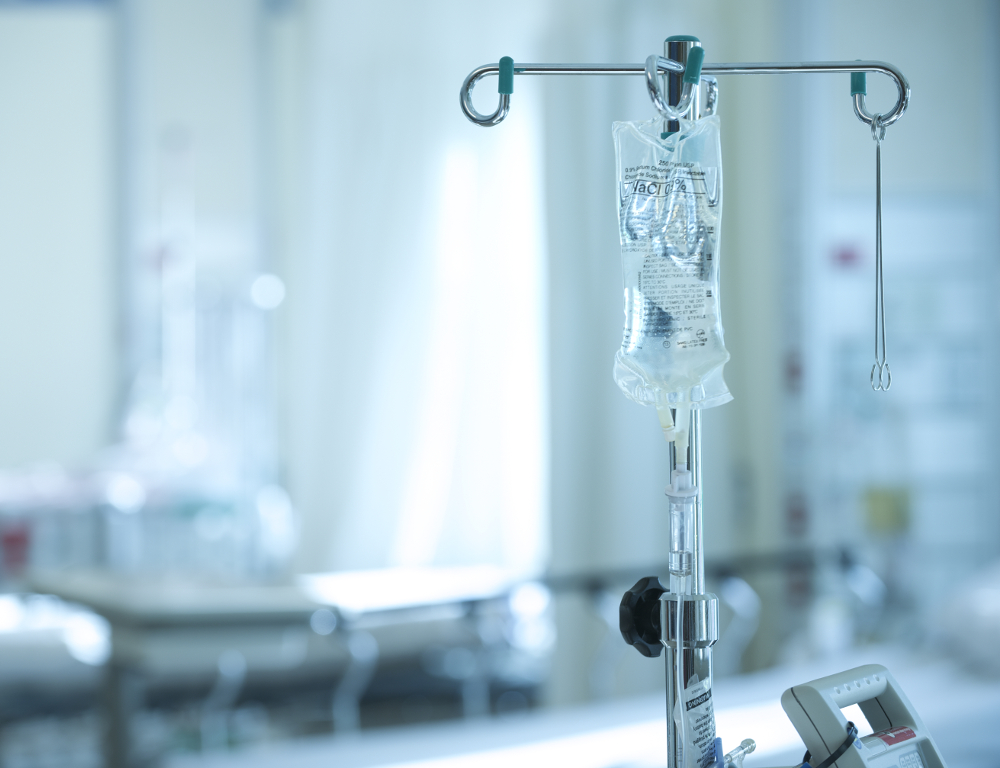
When Hurricane Maria hit Puerto Rico in September, it not only wreaked havoc on the island, it disrupted the production of saline solution bags used to administer fluids and medicine to patients. Now hospitals throughout the U.S. are scrambling to find alternate supplies or devise other ways to administer medicine usually given through IV bags.
Drug and supply shortages like this, however, are not uncommon, and Penn State Health Milton S. Hershey Medical Center is proactively working to address the situation, said Dr. Margaret Mikula, chief quality officer.
The goal is to control demand for products. The Medical Center is still meeting the needs of its patients and doing it in a safe and effective manner. “We are on top of it,” Mikula affirmed. “We are working to conserve.”
The Medical Center has pharmacy and supply chain employees dedicated to acquiring medical supplies during shortages such as these. They are looking at what is available on the market on an hourly basis.
Hospitals nationwide are looking at alternative ways to deliver medicine, including via syringe or pill form. The alternative methods that the Medical Center is using will not affect patients' quality of care or safety. “They are just as effective,” said Vincent Lacroce, administrative director of pharmacy. If patients require IV pain medication, then they will receive them, said Lacroce. The Medical Center is constantly seeking out alternative sources of IV fluids, while also enacting conservation processes. These processes include eliminating waste by using only the exact amount of IV fluids that is medically necessary and using oral hydration and medications whenever possible.
Baxter International, the largest producer of saline solution bags, with three manufacturing plants in Puerto Rico, lost power when the hurricane hit in September and has been running on backup generator power ever since.
Two other pharmaceutical device companies that supply IV bags are also having issues. B. Braun Medical is currently under investigation by the U.S. Food and Drug Administration (FDA) for leaky and moldy IV bags, and ICU Medical is struggling to keep up with the increased demand. For now, the FDA has allowed Baxter to import “mini-bags” from Europe and Australia to help with the shortage.
If you're having trouble accessing this content, or would like it in another format, please email Penn State Health Marketing & Communications.
