Researcher develops method for mapping brain cell change, development in mice
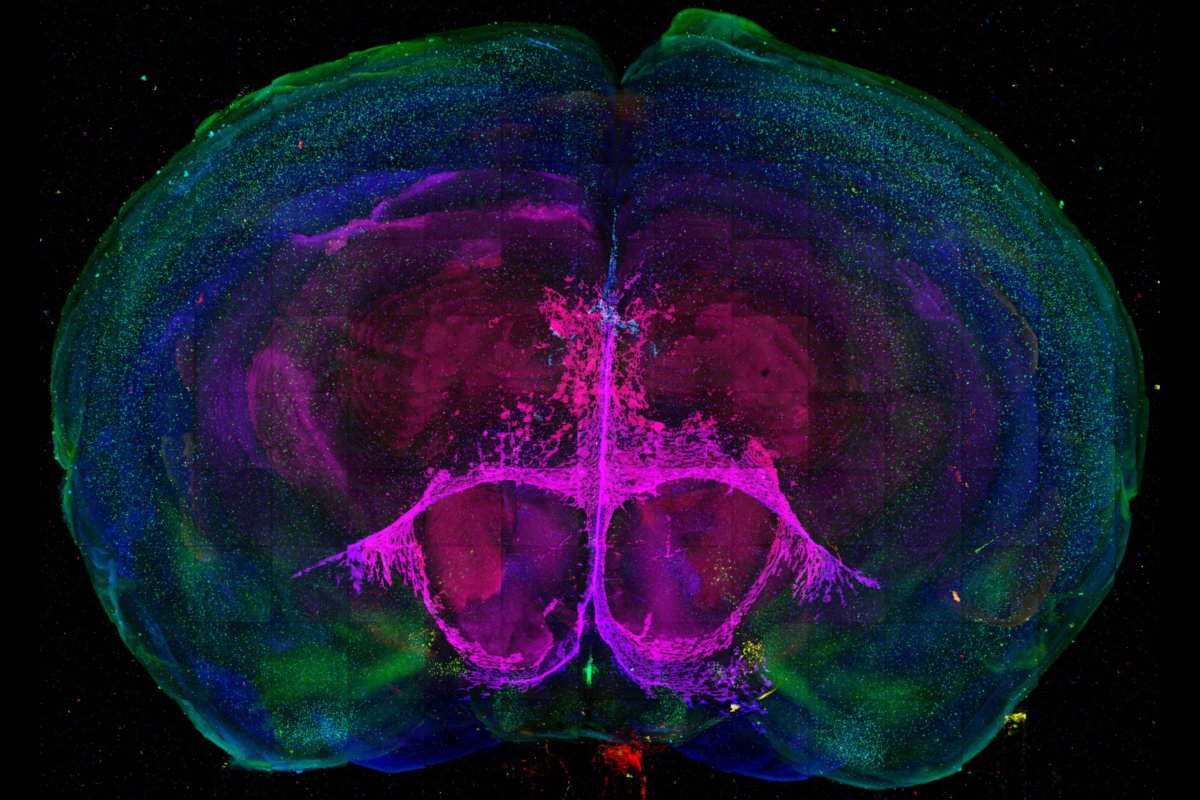
Penn State researchers have developed a new method for studying key moments in brain development. Yongsoo Kim, assistant professor of neural and behavioral sciences at Penn State College of Medicine, is using the method to understand how oxytocin receptor expression changes in normally developing mice and mouse models of autism spectrum disorder.
The technique allows scientists to create maps of developing mouse brains that can display how much of certain cell types are present in different regions – and it is a critical first step in being able to study neurodevelopmental disorders in the brain.
“Key neural connections form during early development,” said Kim. “We can apply this mapping method to study changes of different brain cell types in developing mouse brains to understand neurodevelopmental disorders at a cellular level.”
Kim and his colleagues created maps to help them understand how oxytocin, a neurotransmitter produced by the brain, is utilized by the brain during the course of early development. Previous research revealed that oxytocin plays a role in regulating social behavior, but there is little information on how the receptors that mediate oxytocin’s effect across the brain’s neural networks are present in different parts of the brain across time during development.
The research team hypothesized that different brain regions would have different expression levels of oxytocin receptor (OTR) as individual brain areas mature. Previous studies investigating oxytocin receptor expression used methods where only select regions of the brain could be analyzed in portions. Kim’s new technique is able to image whole mouse brains at a cellular resolution using serial two-photon tomography and machine learning-based algorithms to detect fluorescently-labeled neurons that express oxytocin receptors.
The team created template brains from different early postnatal development periods – 7, 14, 21 and 28 days after birth. The templates were created by generating averages of brain images and labelling key anatomy. They served as a reference point for imaging, detecting, and quantifying the oxytocin receptors during different phases of development.
The results of the study were recently published in Nature Communications. Kim and colleagues found that OTR expression reached its peak in mouse brains 21 days after birth, which is equivalent to early childhood in humans. OTR expression in the hypothalamus continued to increase until adulthood, indicating that oxytocin signaling may play a role in generating sex-specific behavior. They also studied mice who were unable to produce oxytocin receptors and noted that there was significantly reduced synaptic density – indicating that oxytocin plays a key role in wiring the brain.
Kim says the same method used to study OTR in this study could be applied to other brain cell types in order to understand their spatial arrangement across time. The research team built a web-based platform to host and display the new images for other researchers to access.
“These images will serve as an essential baseline to compare OTR expression in various mouse models of brain disorders,” Kim said. “We were able to study OTR expression in a mouse model of autism and will build on these findings by further studying functional and anatomical changes of different brain cell types and how genetic and environmental factors may affect brain development in early childhood.”
Members of the research team include Kyra Newmaster, Zachary Nolan, Uree Chon, Daniel Vanselow and Abigael Weit from the College of Medicine; Manal Tabbaa and Elizabeth Hammock from Florida State University; and Shizu Hidema and Katsuhiko Nishimori of the Fukushima Medical University and Tohoku University Graduate School of Agricultural Science. Newmaster and Nolan are students in the college’s MD/PhD Medical Scientist Training Program. Newmaster is in her third year of graduate study in the Neuroscience PhD, and Nolan is in his fourth year of graduate study in the Biomedical Sciences PhD.
This project was supported by funds from the National Institutes of Health, Tobacco CURE Funds from the Pennsylvania Department of Health, the Japan Agency for Medical Research and Development, and JSPS Grant-in-Aid for Scientific Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or other funding agencies.
If you're having trouble accessing this content, or would like it in another format, please email Penn State Health Marketing & Communications.
