Researchers assess heart failure treatment that ‘closes off’ damaged portion of heart, avoids open heart surgery
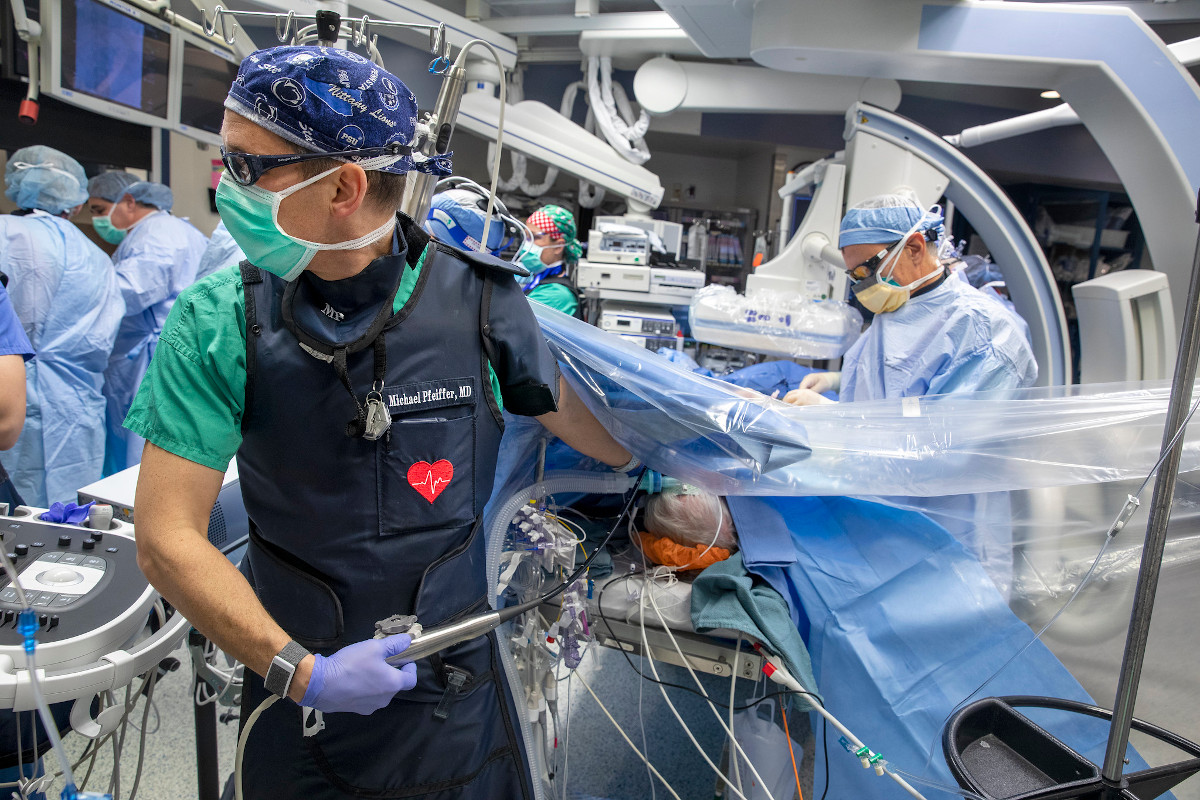
Cardiologists, cardiac surgeons and researchers at Penn State Health and Penn State College of Medicine are investigating a less invasive treatment for heart failure, which affects 6.2 million adults in the U.S. They hope the procedure will improve quality of life for patients. The novel therapy does not require open heart surgery and involves excluding damaged heart tissue, caused by previous heart attacks, from the rest of the heart so it can work more efficiently.
Heart disease is the leading cause of death in both women and men in the U.S. and accounts for about one in every four deaths, according to the Centers for Disease Control and Prevention. Heart failure occurs when the heart is unable to adequately pump blood and oxygen to the body in an efficient way and can develop after a heart attack. It causes fatigue, shortness of breath and other physical limitations for patients. Current treatment options for patients with worsening heart failure despite optimal medical therapy include open heart surgery for the implantation of a left ventricular assist device or heart transplantation – both invasive options.
Dr. Michael Pfeiffer, a cardiologist at Penn State Heart and Vascular Institute and Penn State Health Milton S. Hershey Medical Center, leads a team investigating LIVE (Less Invasive Ventricular Enhancement) Therapy, which is designed for patients who have had a heart attack and suffer from heart failure who are not yet candidates for the more invasive treatments or who want a less invasive alternative.
“Participating in the ALIVE Trial enables us to be on the front lines of research into the most innovative therapies with potential to help patients in central Pennsylvania and beyond who suffer from the limitations of heart failure,” Pfeiffer said. “We are excited about the prospect of treating patients in a less invasive way that may help them avoid some of the risks inherent to open heart surgery and afford them a quicker recovery.”
LIVE Therapy uses the BioVentrix Revivent TC TransCatheter Ventricular Enhancement System and is the first catheter-based procedure designed to close off the damaged portion of the heart caused by a previous heart attack so the healthy portion can pump better and bring more blood and oxygen to the rest of the body. Improving the heart’s ability to pump blood has been linked to better heart function, quality of life and life span.
Click on image below to scroll through photo gallery:
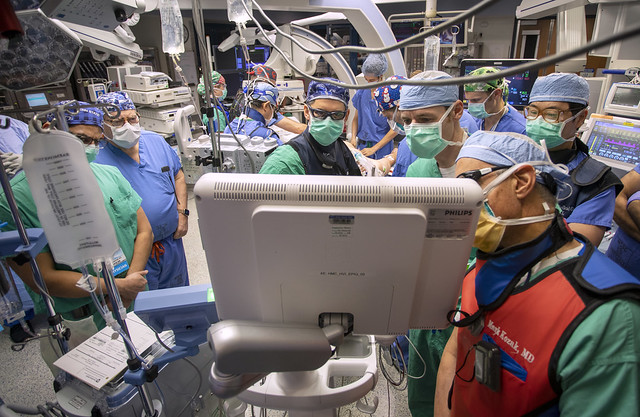
To date, Hershey Medical Center has performed the procedure on 2 patients, the first on December 17, 2020. Pfeiffer said that these and other patients enrolled will be followed over time as researchers evaluate whether the therapy improves heart failure symptoms, quality of life and exercise capacity. They will also investigate whether the therapy prevents hospital readmissions and is safe. Hershey Medical Center is one of 25 U.S. centers participating in the study, which expects to enroll 126 patients in this institutional review board-approved protocol.
“At Hershey Medical Center, we’re fortunate to have access to a broad range of expertise and skillsets that make our participation in this leading-edge research possible,” Pfeiffer said. “Structural heart and research coordinators, cardiologists with expertise in heart failure, imaging and interventional procedures and our cardiothoracic surgeons and anesthesiologists all play an important role in bringing this innovative, investigational treatment to our patients.”
Patients who have had a heart attack and continue to suffer from heart failure symptoms can call 717-531-5967 or PSHVICTO@pennstatehealth.psu.edu to determine if they are a candidate. More information about this trial can be found on clinicaltrials.gov.
For more available clinical trials for cardiovascular disease, please visit Studyfinder.
This research is being conducted using funds from BioVentrix. This research utilizes the Revivent TC™ Transcatheter Ventricular Enhancement System provided by the aforementioned sponsor(s). The Pennsylvania State University does not endorse, promote or recommend the Revivent TC™ Transcatheter Ventricular Enhancement System or any other commercial product or service. The views and opinions expressed by the researchers do not necessarily reflect those of The Pennsylvania State University, and any use of the names and logos of The Pennsylvania State University or its personnel without prior written consent is prohibited.
If you're having trouble accessing this content, or would like it in another format, please email Penn State Health Marketing & Communications.
