Researchers receive $4 million to develop pumps for patients born with heart defects
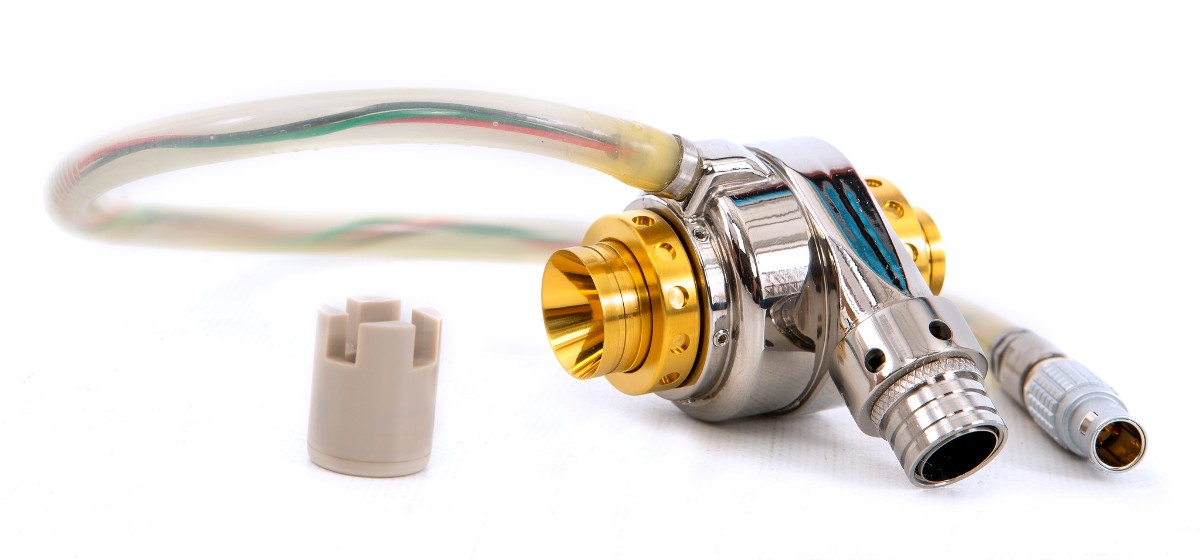
A new heart device in development at Penn State College of Medicine could someday help people born with a serious heart defect. The researchers working on the project received more than $4 million from the U.S. Army Research and Development Command to design a wireless pump that will act as the missing right ventricle in certain people born with heart defects.
As many as 1,200 babies are born each year in the United States with only one functional ventricle due to congenital heart defects. Blood without oxygen normally returns to the heart to be transported to the lungs for oxygen via the right ventricle. However, in patients born with only one ventricle, surgeons perform a Fontan procedure, where de-oxygenated blood bypasses the heart and flows directly to the lungs to receive oxygen before returning to the heart to be pumped to the rest of the body by the single ventricle.
Survival rates for babies who receive the Fontan procedure have improved over the past few decades, but as these patients age into young adults, they may experience serious complications that impact their quality of life including fluid retention, swelling, abnormal cardiac output and liver failure. William Weiss, Howard E. Morgan Professor of Surgery in the College of Medicine’s Division of Applied Biomedical Engineering, is leading a project to develop a device that he hopes will help prevent these complications.
“Finding long-term solutions for these patients is critical,” said Weiss. He noted that heart transplantation is a poor option due to limited donor hearts and prior surgeries and blood transfusions that produce antibodies to donor tissue. “We envision a device that essentially acts as the right ventricle and will pump blood to the lungs to receive oxygen and expel carbon dioxide.”
Although left ventricular assist devices are available for long term support in patients with left ventricular failure, there are currently no devices available for long term support or replacement of the right ventricle. According to Weiss, currently available pumps have electrical cables running through the skin, which is not ideal for long-term use and poses an infection risk. The team envisions a completely wireless system, based on a system they designed for pulsatile pumps that was successfully tested in patients.
Weiss and other team members completed the first phase of the project, which included engineering and designing the small pump. Since blood is a unique liquid with special properties, they conducted a series of tests to see whether the device could move blood effectively and also how blood would interact with the surface of the pump, to avoid clotting or other health issues. While initial implants in animal studies showed good results for safety and efficacy, Weiss said there’s still more work to be done.
The next phase of the project will focus on developing an implantable, wireless controller for the pump, which will require additional testing. Although it will be years before the device is ready for clinical testing in human subjects, Weiss remains optimistic about the progress.
“If we’re successful, thousands of aging Fontan patients will have a new option that could last ten years or longer,” Weiss said. “We want to improve their quality of life. One day at a time, we’re working towards that.”
Weiss is a member of the College of Medicine’s Division of Applied Biomedical Engineering — a group of experts from diverse disciplines dedicated to the design, manufacture and testing of artificial hearts and heart devices. Penn State has been an international leader in the research, development and clinical use of heart pumps and artificial hearts since the 1970s when Dr. John Waldhausen, the Department of Surgery’s founding chair, invited Dr. William Pierce to join the new faculty. Since then, College of Medicine researchers have partnered with peers across the globe — including colleagues from Penn State College of Engineering — to develop total artificial hearts, left ventricular assist devices and other cardiovascular devices.
Weiss discloses receipt of royalties and licensing fees for wireless technology patents licensed to Minnetronix Inc. by Penn State Research Foundation.
Learn more about artificial heart research history at the College of Medicine.
Learn more about heart and vascular clinical trials at Penn State College of Medicine.
EDITORS: There are galleries of high-resolution, downloadable photos available for your use. A gallery of scientists creating artificial heart parts at College of Medicine laboratories is available here. A gallery of historic and current heart devices developed by Penn State researchers is available here.
If you're having trouble accessing this content, or would like it in another format, please email Penn State Health Marketing & Communications.
