Flow cytometry helps unveil Schweinfurthins’ unique strategy for exposing melanoma cells
Identifying new therapies that effectively treat cancer is critical to address unmet clinical needs. A derivative of the family of natural products called schweinfurthins uniquely induce cancer cell death, according to a recent study by Penn State College of Medicine and Penn State Cancer Institute researchers.
The same researchers previously found that these compounds improved immunotherapy of malignant melanoma in a preclinical model and induced immunogenic cell death (ICD) in the melanoma cells. This type of cell death alerts and recruits the immune system to respond to cancer. Defining how schweinfurthin compounds induce ICD was the focus of a recent study performed by Raymond Hohl, MD, PhD, director of Penn State Cancer Institute, and colleagues.
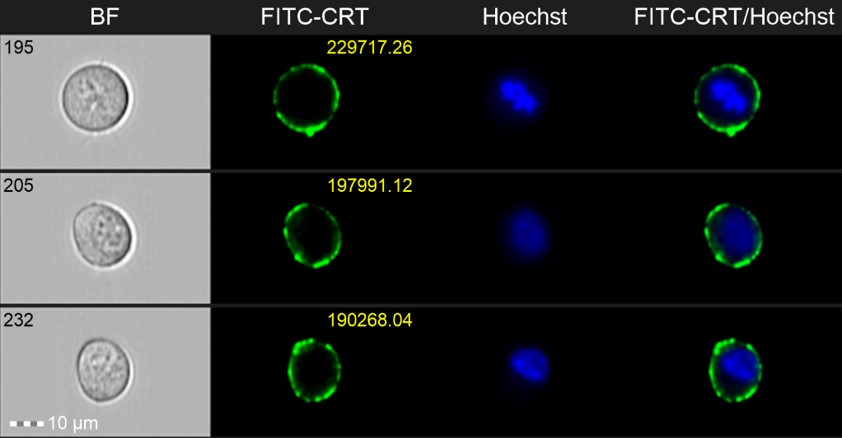
The investigators used a combination of approaches, including traditional flow cytometry and imaging flow cytometry to define the molecular mechanisms that induce ICD in melanoma cells following treatment with 5’-methoxyschweinfurthin G (MeSG). Imaging flow cytometry combines traditional high-throughput flow cytometry analysis with image capture to reveal cell morphologic characteristics and enable localization of fluorescently labeled markers. Imaging flow cytometry revealed the kinetics of calreticulin exposure at the cancer cell surface, a key characteristic of ICD, following treatment with MeSG. This approach enabled the authors to both confirm cell surface localization of calreticulin and identify unique kinetic features of its localization. Imaging flow cytometry was also used to demonstrate that MeSG treated tumor cells were more effectively captured by immune cells called dendritic cells, which are key activators of anti-tumor immunity. The researchers concluded that schweinfurthin‑treated cells are able to trigger anti-tumor immune responses.
The authors further established that the mechanism of ICD induced by schweinfurthins is distinct from that induced by previously investigated drugs. In particular, MeSG did not cause endoplasmic reticulum stress and did not require the protein kinase PERK to induce calreticulin exposure. Caspase inhibitors partially rescued tumor cells from MeSG-induced cell death, but failed to reduce calreticulin exposure. However, an intact ER to Golgi transport system was required for calreticulin exposure. Research is ongoing to define the key molecular pathways involved in this process. The results were published in the journal OncoImmunology in August 2022.
“This is a great example of the kind of transformative data that these shared resources allow,” said Jeffrey Neighbors, PhD, a co-investigator on the study. “We would not have had the ability to acquire an instrument with these capabilities for our own labs, and even working on a highly collaborative project with several PI’s, the cost of this level of sophisticated instrumentation is prohibitive. It is exactly this type of capability that make the Flow Cytometry Shared Resource so valuable.”
Ruoheng Zhang and Todd Schell, PhD from the Penn State College of Medicine and Penn State Cancer Institute also contributed to this research. Raymond Hohl and Jeffrey Neighbors have financial and ownership interest in Terpenoid Therapeutics Incorporated and IOThera Incorporated which provided the schweinfurthin compound for these experiments.
This research was sponsored by a Penn State University Professorship in Medical Oncology, the Miriam Beckner Cancer Research Endowment and a gift from Highmark to the Penn State Cancer Institute.
The research was supported by the Flow Cytometry Core Facility (RRID:SCR_021134). The ImageStream X Mk II Imaging Cytometer was funded, in part, by a grant from the PA DOH using Tobacco CURE Funds. The Department specifically disclaims responsibility for any analyses, interpretations or conclusions.
If you're having trouble accessing this content, or would like it in another format, please email the Penn State College of Medicine web department.
