Winter 2017 edition: Sports Medicine for the Primary Care Provider

The Winter 2017 edition of Sports Medicine for the Primary Care provider was recently published. Articles in this edition include “Platelet-Rich Plasma to Treat Musculoskeletal Injuries,” “Help Patients Keep Their New Year’s Resolutions” and “Shoulder Instability Issues.”
A printable version of this newsletter is available here.
All articles also appear below.
Welcome
Dear Health Care Provider,
My name is Matthew Silvis, medical director of Penn State Primary Care Sports Medicine. Below is the winter edition of our Primary Care Sports Medicine Newsletter, a biannual newsletter of seasonal sports topics. We hope you find the information useful and appreciate any feedback you have to enhance our efforts. We have selected a variety of topics for this issue.
If you’d like to receive this newsletter by email, please send your email address to my administrative assistant, Tracie Kirkessner, at tkirkessner@pennstatehealth.psu.edu. Please send any future topic ideas to Tracie or to me at msilvis@pennstatehealth.psu.edu.
Enjoy,
Matthew Silvis, MD
Associate Professor
Penn State Health Milton S. Hershey Medical Center
Platelet-Rich Plasma to Treat Musculoskeletal Injuries
By Ryan Norton, DO, Primary Care Sports Medicine fellow, Penn State Health Milton S. Hershey Medical Center
Overuse injuries to muscles, tendons and cartilage in active patients can be quite difficult to treat. Surgical options are often limited and a portion of this population does not respond to conventional therapies, such as activity modification, physical therapy and anti-inflammatories.
Furthermore, commonly used glucocorticoid injections are not without risk and play a very limited role in actually rebuilding the damaged tissue. To return individuals to activity and minimize time-loss from training and competition, the medical community continues to search for a safe and effective regenerative therapy.
Platelet-rich plasma (PRP) is increasing in popularity. Extracted from the patient’s own blood (autologous), PRP is defined as blood plasma that contains a platelet count above the patient’s baseline. Although its first non-cardiac use was reported in 1998 to enhance the healing of bone grafts following oral surgery, the injection rapidly gained popularity in the U.S. in 2011 after high-profile athletes Tiger Woods and Kobe Bryant received the injection.
PRP is usually injected in an office-based setting under ultrasound-guidance to ensure precise delivery. Most offices are purchasing PRP “kits” that contain the supplies needed to perform them. Venous blood is drawn in typical fashion. The sample is then centrifuged to separate out the various components. The desired supernatant containing plasma, PRP and growth factors settles on top and is withdrawn using a simple needle and syringe. The platelet-rich plasma, measuring between 2-5 ml on average, is then injected into the injured tissue. The entire process, from phlebotomy to injection, takes about 45 minutes and is performed in a single extended outpatient visit.
The basic science behind PRP relies on the body’s innate healing ability. Platelets contain several biologically active proteins, or growth factors, that are critical to tissue healing. Additionally, platelet-rich plasma (once activated in vivo) creates fibrin scaffolding upon which cell development and eventually tissue repair can occur.
Evidence supporting PRP remains limited and clinical trials have yielded mixed results.
There is increasing and evolving evidence that PRP may be beneficial in the treatment of common extensor (lateral epicondyle) tendinopathy, jumper’s knee (patellar tendon) and knee osteoarthritis in patients who do not respond to conventional therapies. On the contrary, clinical studies have not been able to clearly demonstrate a benefit in other indications, such as Achilles tendinopathy, plantar fasciitis, rotator cuff pathology and hip osteoarthritis. Randomized, prospective placebo-controlled clinical studies are needed to determine the appropriate use of PRP.
There are a number of variables that likely impact a patient’s response to PRP and complicate research efforts. For example, the platelet concentration in a given injection can range from 1.5 times up to 6 times the normal concentration. Increasing concentrations may actually prohibit PRP’s effect, thereby making this a critical area of study. Additional variables being investigated include the potential added benefit from fenestration (needling the tissue prior to injecting), the number of injections needed (up to three have been studied), and whether including leukocytes in the plasma is detrimental to the biological response.
There is very little risk associated with PRP injections. Any time the skin is disrupted there is a risk of bleeding and infection. Injecting a load-bearing tendon, such as the Achilles, carries a theoretical risk of rupture; clinicians must consider whether to off-load the injected tissue for a week or two, to minimize this risk.
For now, PRP is considered experimental and not covered by most insurance companies. At Penn State Bone and Joint Institute, our primary care sports medicine providers use a kit from Regen Lab. The patient is charged the manufacturer’s charge of one kit. The cost to receive the injection varies widely across the country, with some patients reportedly paying thousands of dollars.
Platelet-rich plasma is a promising treatment for musculoskeletal injuries. Despite PRP’s status as an experimental therapy, it is a reasonable option for patients who have failed conventional therapies and are eager to return to an active lifestyle.
Help Patients Keep Their New Year’s Resolutions
By Shawn Phillips, MD, MSPT
The New Year is a time when gym memberships are on the rise as people work to burn off the holiday calories they just put on. Unfortunately, studies show that many of these memberships go unused and most people give up on exercise within a few weeks. The reasons for this are multifactorial, but one reason is that patients are not prepared or properly educated on exercise prior to beginning a program. Primary care providers can play a part in helping patients achieve their exercise goals. A recent CDC report shows that exercise is discussed in fewer than one-third of all health care visits. So how can we help our patients to get active?
Preparing for exercise
It is important for health care providers to assess a patient’s baseline level of fitness. Traditionally, exercise stress testing was recommended for patients over age 40 before starting a new exercise routine. This type of testing, however, often scared patients and became a barrier to starting a program. Current recommendations are that little to no testing is required for patients to initiate a light to moderate exercise program. An asymptomatic patient with known risk factors such as diabetes, cardiovascular disease or chronic kidney disease does not require stress testing to start a light to moderate exercise program. Setting a follow-up appointment with patients to discuss their exercise helps ensure they are exercising safely and can also bolster compliance. Symptomatic patients including those with chest pain or shortness of breath at rest or with minimal activity, significant lower extremity edema, signs of claudication, palpitations or syncope should obviously be evaluated more thoroughly before starting an exercise program.
Exercise prescription
Many primary care providers are uncomfortable prescribing exercise in specific detail. The American College of Sports Medicine (ACSM) recommends that adults achieve 150 minutes of moderate or 75 minutes of vigorous exercise per week. Examples of moderate exercise include walking at greater than 3 miles per hour, light cycling or ballroom dancing. Examples of vigorous exercise include running, swimming, singles tennis, cycling greater than 10 miles per hour and aerobic dancing. Unfortunately simply giving patients this information is often not enough to help them maintain their exercise program. For our sedentary patients, 150 minutes of exercise per week seems daunting. We also do not want to give patients the idea that exercising at a rate less than this goal is ineffective.
Prescribing exercise to our patients will include four domains: intensity, frequency, time and mode.
Intensity is a measure of exertion. Moderate exercise intensity is defined as exercising at 60 to 80 percent of maximal heart rate. Instructing patients on heart rate calculations can create undue stress and work for the patient. Practically, we can instruct patients on utilizing the “talk test” to mark their intensity. For patients whose goal is to exercise with moderate intensity, they should be able to carry on a conversation, needing to stop every 3 to 4 words for a breath. Exercising vigorously, a patient will need to pause with each word.
Frequency is a measure of how often one exercises. Our goal should be to have our patients exercising most days per week, though two to three days per week might be a nice start for our more sedentary patients.
Time goals should be individualized. Reaching 150 minutes of exercise per week can be achieved in many ways and as little as 10 minutes of exercise per session can be beneficial. A goal for our sedentary patients may be to exercise 10 minutes per day, three days per week. This can be increased gradually over time. A barrier to exercise for many patients is not having enough time. Providers can help patients find ways to work exercise into their daily routine. Patients can walk for 10 to 15 minutes during work breaks. Having patients park their car a 10-15 minute walk from their workplace will help them achieve 20 to 30 minutes of exercise each day as part of their commute. In a similar way, biking to work or other means of active transportation can help patients work exercise into their day without adding another checkbox onto the to-do list.
The last domain to consider is mode, or the activity itself. Many patients find going to the gym or walking on a treadmill boring. It is important for providers to talk with patients about what they enjoy and set their exercise prescription goals accordingly. For some patients, it's playing pick-up basketball. For others it may be yoga. For some it is activities, such as gardening. Still others enjoy dance aerobics, ballroom dance class or simply walking the golf course instead of using a cart. Helping patients find an activity that they enjoy will go a long way to helping them get and stay fit. Joining a gym or buying expensive exercise equipment is not required to maintain an active and healthy lifestyle.
Making a referral
Some patients may want or need more guidance and observation when initiating an exercise program. Many personal trainers at local fitness clubs are not well-suited to counsel patients with medical issues. A referral to an exercise physiologist or physical therapist may be helpful. Here at Hershey, Penn State offers the FitRx program as a resource for providers. We can refer patients directly to this program, which includes an intake evaluation and development of an exercise program by an exercise physiologist. Cost for this program is similar to many gym memberships and is partially covered by some insurance programs.
Finally, it is important to remind patients that fitness is the ultimate goal. While exercise may contribute to weight-loss goals, patients who wish to lose weight will often be disappointed if not losing weight. It is important to remind patients that exercise, even in the absence of weight loss, can have significant health benefits.
The ACSM’s Exercise is Medicine website can be a valuable resource for providers who wish to counsel their patients on exercise. Remember too, physicians who exercise themselves are more likely to counsel their patients on exercise, so let’s get moving!
Shoulder Instability Issues
By Rob Gallo, MD
The winter sports season has hit full swing. The first wave of injuries is trickling into training rooms, acute care centers and primary care offices. Among the most common injuries sustained in winter sports are shoulder dislocations and subluxations. NCAA injury surveillance studies estimates that shoulder dislocations occur at a rate of 0.12 per 1,000 hours exposures, including games and practices. In fact, two winter sports, wrestling and ice hockey, carry the highest susceptibility to shoulder instability episodes according to these studies. Given the relatively high rate of shoulder instability, most team physicians encounter a winter sports athlete who sustains a shoulder dislocation. Here are some commonly encountered questions to safely manage athletes with shoulder instability.
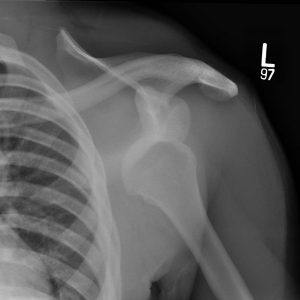 What kind of sling should be used to immobilize athletes after a shoulder dislocation?
What kind of sling should be used to immobilize athletes after a shoulder dislocation?
The optimal position of immobilization following an anterior shoulder dislocation remains controversial. Traditionally, athletes are placed into a simple sling or immobilizer that allows for shoulder adduction and internal rotation. However, MRI studies by Itoi, et al., demonstrated that the anteroinferior labrum, the structure most commonly torn after an anterior shoulder dislocation, assumes a more anatomical position with the shoulder in external rotation. Clinical studies verified the anatomic studies: Itoi, et al., found a 46.1 percent relative risk reduction in shoulder dislocations among a group that was immobilized with the shoulder in external rotation. Studies independently verifying Itoi's work have been lacking. In fact, a recent prospective, randomized study by Liavagg, et al., failed to show a significant reduction in the re-dislocation rate when immobilized in external rotation. Based on these findings, clinicians must weigh the potential benefits of immobilization in external rotation with the availability and ease of use with traditional slings.
How long should athletes be immobilized after shoulder dislocation?
Limited data exists to guide clinicians on the optimal length of immobilization following shoulder dislocation. A prospective randomized study by Hovelius, et al., compared the re-dislocation rates at two years among those treated with an immobilizer for three to four weeks and those allowed early mobilization. In this study of 257 patients, the authors found no significant difference in the re-dislocation rate between the two groups. Therefore, most prescribe a sling for comfort and encourage early range-of-motion and strengthening, as soon as tolerated.
What imaging should be obtained after a shoulder instability episode?
Most agree that a standard shoulder series including anteroposterior, Grashey (true AP), scapular Y, and, most importantly, axillary lateral radiographs should be obtained pre- (if possible) and post-reduction to confirm concentricity of the humeral head within the socket and assess for any fractures of the humeral head or glenoid. Humeral head fractures in young athletes are usually impaction fractures that involve the posterolateral aspect of the head (Hill-Sachs fracture), while fractures of the glenoid often involve the anteroinferior quadrant of the glenoid (bony Bankart lesion). Bony Bankart fractures involving as little as 15 percent of the glenoid surface may render the shoulder joint grossly unstable and may benefit from prompt surgical fixation.
Advanced imaging, such as CT scans and MRI arthrograms, are useful for surgical planning to completely define the extent of the osseous and soft-tissue injuries. Most anterior shoulder dislocations involve a disruption of the anteroinferior labrum from the glenoid. And – typically renders the anterior band of the inferior glenohumeral ligament, the primary restraint to anterior movement of the humeral head, unstable. Often, an MRI with intra-articular contrast is preferred to increase the sensitivity of detecting this injury. CT scans, particularly those with three-dimensional reconstruction, can further elucidate severity of bony injuries. Large bony defects can result from repeated dislocations and are a leading cause of re-dislocation following surgical fixation. Therefore, those with large bone defects often require more complex bone grafting techniques.
Following a shoulder dislocation, when can athletes return to play?
In-season, return to play following a shoulder dislocation is possible in those athletes without significant bone loss. Return to athletic endeavors after non-operative management is permissible following a brief period of immobilization and early rehabilitation focusing upon restoration of full range-of-motion and strength. To be permitted to return to in-season play following a shoulder dislocation, the athlete must meet the following criteria: (a) symmetric, pain-free shoulder motion and strength, (b) ability to complete sport-specific skills, (c) lack of subjective or objective instability.
For those who wish to undergo surgical stabilization, return to play is usually not considered for at least four to six months post-operatively, depending upon the surgeon, extent of injury and sport.
Are braces helpful to prevent recurrence of shoulder instability once the athlete returns to play?
There are a variety of braces available to assist athletes return to play. Most braces function by either restricting motion or improving proprioception. More restrictive braces, such as lace-up braces (Sawa) and braces that attach to shoulder pads, tend to decrease the risk of an instability event, but are often poorly tolerated due to their deleterious effects on athletic performance. Many athletes, especially overhead athletes, prefer neoprene-type braces which allow more range-of-motion. Despite theoretical benefits of bracing, there is limited data to suggest that these braces decrease dislocation rates.
What can be expected after a shoulder dislocation?
The chances of an athlete suffering a second dislocation range from 39 to 94 percent (8-12). Age younger than 20 years, contact sports, those with global hyperlaxity and male athletes tend to have a higher rate of recurrent instability events. Repeated shoulder dislocations can cause progressive damage to the articular cartilage, increased laxity of the surrounding ligaments and joint capsule, and erosion of the bone structures. Bone-loss threatens the integrity of the joint and leads to increased instability and more extensive surgical reconstruction.
Contact Us
Learn more about the Sports Medicine team at Penn State Health here.
If you're having trouble accessing this content, or would like it in another format, please email the Penn State College of Medicine web department.
