Unique division brings heart devices to life
An engineer, a surgeon, and a machinist walk into a conference room.
It might sound like the start of a bad joke, but it’s a regular scene in Penn State Hershey’s Division of Artificial Organs, where experts in vastly different fields bring their knowledge together to design, manufacture, implant and test artificial hearts in one location.
Cardio-thoracic surgeon Dr. William S. Pierce formed the team in 1970 when he came to Penn State’s then-new Milton S. Hershey Medical Center after working on artificial heart development for the National Institutes for Health. Penn State’s strong engineering staff and Hershey’s suburban location offered the resources to develop the kind of collaborative program he envisioned.

Eric Yeager makes blood sacs in Penn State Hershey’s Division of Artificial Organs by dipping polished stainless steel molds into honey-colored liquid polyurethane polymer.
Forty-five years later, Dr. Gerson Rosenberg, chief of the Division of Artificial Organs, can walk down the hall from his office to a machine shop, plastics lab, metal-polishing station and rooms where mock circulatory testing is done on heart-assist devices for adults and children. An assist device helps a sick heart do its work so it can rest while the patient awaits a transplant, so researchers are always looking for ways to improve the devices to work better and for longer.
<<View a video of Dr. Piece and Dr. Rosenberg’s work>>
At a nearby facility, veterinarians provide pre- and post-op care for animals implanted with pediatric heart-assist devices and a new pneumatic heart pump — operated by air pressure — that could improve the lives of young adults and adolescents born with congenital heart defects.
“We are unique in that everything from start to finish is done in one location,” Rosenberg said.
The artificial organs program – known worldwide for developing the Arrow LionHeart, the first totally electrical, fully implantable heart assist device – is now charged with developing the pneumatic devices for children and young adults with heart problems.
It is the only operation of its kind in the country, Rosenberg said.
<<View a photo album of work currently being performed by the Division of Artificial Organs>>
In the machine shop, designer Ray Newswanger creates digital models according to Rosenberg’s specifications.
“We come up with ideas to meet the need,” Newswanger said. “It’s open to your imagination what we could create here.”
Machinist Patrick Leibich works with both manual and computer-controlled lathes and milling machines to cut pieces of pumps and valves from solid stock titanium, stainless steel and several types of medical-grade plastics. Leibich uses his knowledge of the properties of different materials to select the appropriate method and equipment to manufacture the components that will eventually be grinded, welded, polished and assembled according to stringent requirements.

Machinist Patrick Leibich works with both manual and computer-controlled lathes and milling machines to cut pieces of pumps and valves from solid stock titanium, stainless steel and several types of medical-grade plastics.
Next door in the polymer lab, plastics specialist Eric Yeager makes blood sacs by dipping polished stainless steel molds into honey-colored liquid polyurethane polymer. With properties similar to Spandex and Lycra, the polymer provides enough elasticity for the sacs to stretch without ripping. One dip takes 30 minutes, so gas bubbles don’t form. It then must cure for two hours before it can be dipped again. The process repeats six more times.
“It takes several days to get a batch,” Yeager said.
Brad Doxtater winds plastic threading onto a thin tube by hand, then evens it out with a machine to create a silicone sealed vascular graft that connects the blood pump to the patient’s heart. He assembles and carefully removes any air trapped between the blood sac and air diaphragm in a pneumatic pump.
“It’s a safety net to prevent the sac from being completely flattened,” he said.
Doxtater is responsible for collecting all the parts that will make up a pump, inspecting and cleaning them before assembling each – one at a time, start to finish, to maintain focus – in a clean room.
In a room the size of a closet, metal specialist Ted Moore spends hours peering through a microscope to polish petite rough frames and tiny discs for the valves that go into the pediatric heart pumps. He punches little polishing wheels from cardboard detergent boxes and sharpens pieces of Q-tips to create homemade tips.
“The ones that are commercially available are not useful for what I am doing,” he said. The attention to detail is not just for cosmetic purposes. “If it’s too rough, the blood will clot on the inside,” he said.
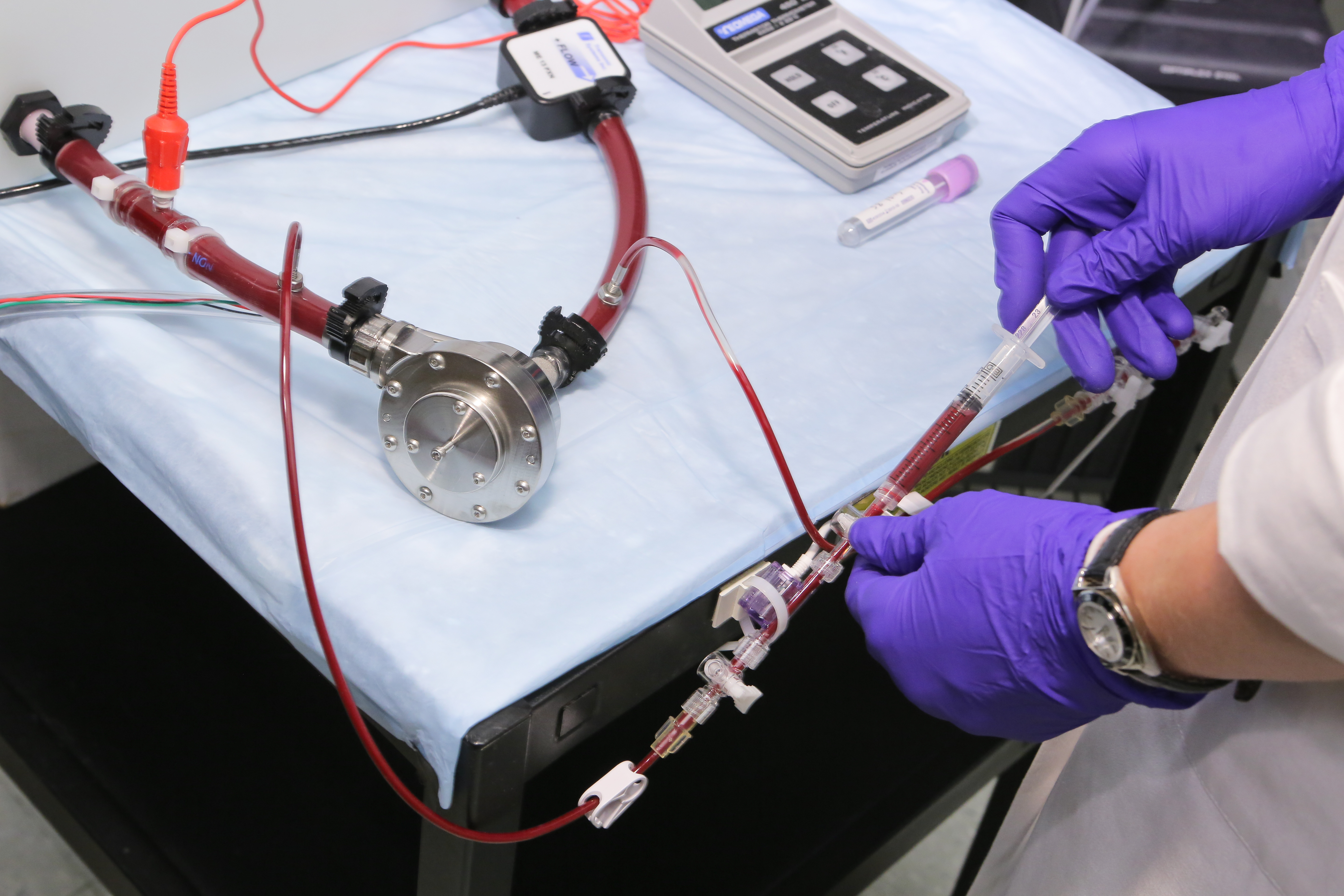
Clotting factors are just one of the things that research assistant Megan Stauffer studies in the mock circulatory laboratories in Penn State Hershey’s Division of Artificial Organs.
Clotting factors are just one of the things that research assistants John Reibson, Branka Lukic and Megan Stauffer study in the mock circulatory laboratories. There, pumps and tubes are filled with a combination of glycerin and water that mimics the thickness of blood and set to run for a certain length of time to test stability of rotors, fluid movement and wear on the parts. Blood is placed between two rotating cylinders of a machine designed to break down red blood cells to measure how much stress can be put on the blood before the all-important cells are destroyed.
In the corner of one lab, the pump and clear tubing of a testing device churn rhythmically over a sink, mimicking a human heartbeat and providing a steady rhythm for the work of the day. A sticky note on the pump notes that it hasn’t missed a beat since May 2010, except for occasional checks for wear on the components. The researchers know that the average human heart beats 50 million cycles each year, so they estimate the testing device has completed about 250 million beats since it was put together.
Designing, creating, testing, then re-designing and retesting artificial organs and heart pumps is a time- and labor-intensive operation.
And it’s not cheap.
“Pre-FDA, medical devices weren’t treated like a drug, so you could make a pump here, write a protocol, send it for review, and if it was approved, you could put it in a patient,” Rosenberg said. “Now, if you want to bring a new LVAD (left ventricular assist device) to market today, it would cost approximately $100 million.”
About a third of the cost of a new medical device is documentation. And nearly a quarter of those who work in the industry exclusively do quality control. Yeager—the plastics specialist—describes the process of making heart pumps as building a castle of playing cards. In his lab alone, three-quarters of the blood sacs he makes are rejected for one reason or another.
“Maybe they have excess particle or they’re the wrong thickness. There are so many things that can take you out,” Yeager said. “You want people who make heart pumps to be under that kind of pressure. But that’s why, when you get to the top and build something that works, you are kind of ecstatic.”
Many other artificial heart programs have come and gone in other parts of the country since Pierce came to Hershey. But like the testing device that continues to beat over its lab sink year after year, the experts who work together in Penn State Hershey’s artificial organs program continue their quest to perfect the next best thing to a strong human heart.
Pierce has an idea why: “I think we have made an effort here to keep things clinically relevant.”
Once research and testing is complete, the work of the Penn State Hershey engineers and scientists is published in the professional literature. Proprietary information – trade secrets that could be patented – remains under university control to attract companies that may want to help bring a new product to market.
The program is ready to partner companies that may be interested in the pediatric heart pump technology they have been working on for so long.
“It’s not a big market,” Rosenberg said. “It has to be more of a philanthropic company that has helping kids as its primary goal.
“Everything we do is aimed at improving these devices and making them attractive to companies so it’s more likely something will reach the market and improve the quality of life for heart patients.”
<<View a photo album of work currently being performed by the Division of Artificial Organs>>
-Jennifer Vogelsong
If you're having trouble accessing this content, or would like it in another format, please email Penn State Health Marketing & Communications.
